Issue 08 (May 5)
COSSA Washington Update, Volume 34 Issue 8
Featured News
COSSA in Action
Congressional News
- NIH Appears Before Senate Appropriations Subcommittee
- House Energy and Commerce Committee Releases Second Version of 21st Century Cures Draft
Federal Agency & Administration News
- ACS to Retain Marriage, Field of Degree Questions Proposed for Elimination
- White House Seeks Input on Using Science and Technology to Improve the Lives of Older Americans
- Eliseo J. Pérez-Stable Named Director of the National Institute on Minority Health and Health Disparities
Funding Opportunities
- NIH: NIMHD Seeking Input for Health Disparities Science Vision
- NIH: Input Sought on Precision Medicine Cohort
Member Spotlight
COSSA and Partners Advocate for Social Science Funding and Sound Scientific Policies
COSSA has joined with its partner organizations and coalitions on several letters to Congress urging increased funding for social and behavioral science programs and sound scientific policies. Recent letters addressing fiscal year (FY) 2016 funding and scientific conference travel can be viewed here.
NIH Appears Before Senate Appropriations Subcommittee
On April 30, National Institutes of Health (NIH) director Francis Collins made his annual appearance before the Senate Appropriations Subcommittee on Labor, Health and Human Services, Education, and Related Agencies (Labor-HHS) to discuss the President’s proposed FY 2016 budget request for the agency. The NIH director was accompanied by several institute directors: Anthony Fauci, National Institute of Allergy and Infectious Diseases (NIAID), Douglas Lowy, National Cancer Institute (NCI), Gary Gibbons, National Heart, Lung, and Blood Institute (NHLBI), Jon Lorsch, National Institute of General Medical Sciences (NIGMS), and Tom Insel, National Institute of Mental Health (NIMH).
Subcommittee Chairman Roy Blunt (R-MO) noted that throughout history the practice of medicine has been largely reactive, waiting until the onset of most diseases before being able to treat them or begin the process of curing them. He further acknowledged that science does not fully understand the genetic and environmental factors that cause major diseases and that treatment is imprecise, unpredictable, and often ineffective. Highlighting the fact that the NIH’s budget request included what he called the “revolutionary concept of precision medicine,” Blunt pledged his support for the President’s proposed Precision Medicine Initiative (PMI) (see Update, April 21, 2015) and announced his intent to prioritize funding for NIH. Despite the challenges that the Subcommittee faces in deciding how to allocate funding, the Committee, Blunt declared, “will be supportive of PMI and the ongoing work of the NIH and the promise it holds for the future.”
Subcommittee Ranking Member Patty Murray (D-WA) called NIH’s work “vitally important” to the effort to keep families and communities healthy. She emphasized that all Americans are touched by NIH-supported research. Sharing that she is deeply troubled by the steady erosion of NIH’s purchasing power over the last decade and is concerned about other areas in the budget as they relate to growing a strong middle class, Murray pointed out the budget resolution recently passed by both the House and the Senate falls short of the funding levels needed to address this need. The President’s budget calls for replacement of the automatic spending cuts, also known as sequestration, which allows for a $1 billion increase for NIH in FY 2016, said Murray.
NIH: “Focusing Intensively on Prioritization of NIH Resources”
Collins began his testimony by noting that the NIH, as a federal agency, is acutely aware that in order to achieve its mission it must serve as effective and efficient stewards of the resources it has been given by the American public. To this end, Collins testified that one of the ways that the agency is accomplishing this objective is by “focusing intensively on prioritization of NIH resources.” He explained that this effort “involves developing and applying advanced methods of portfolio analysis, identifying the most compelling opportunities within each institute and center, fostering creative trans-NIH collaborations, and enhancing use of the Common Fund.”
He stated that to support this focus on priority setting, the NIH is developing an overarching NIH strategic plan—which it will link to the individual strategic plans of the agency’s 27 institutes and centers—that reflect the rapid progress in bioscience. According to the NIH director, the plan will be completed by December 2015. In addition, he noted that the NIH is working “to optimize the peer review process to enhance diversity, fairness, and rigor, and the reproducibility of NIH-supported science.” The agency remains “100 percent committed strengthening and sustaining”the scientists it supports by incentivizing early stage young investigators, revitalizing physician-scientists training, and increasing the diversity of the NIH research workforce, he explained. Collins stressed that the NIH is confident that with those goals it will be able to support the best and brightest ideas, while maintaining “the agency’s core mission and inspiring public trust in the world’s premier biomedical research agency.”
The NIH is excited to take the lead role in the multi-agency Precision Medicine Initiative (PMI), with its near term goal of focusing on cancer, Collins noted. He explained that the longer term goal of PMI includes the launching of the “unprecedented national research cohort of one million or more volunteers who will play an active role in how their genetic, environmental, and medical information is used for the prevention of illness and management of a wide array of chronic diseases.” The objective will be to expand the benefits of precision medicine into “myriad aspects of health and health care.” Participants will share data from electronic health care records (EHRs), results of imaging and laboratory tests, lifestyle data and environmental exposure recording tracked through real-time mobile health devices, and genomic information. This will allow researchers to advance the information “derived from this cohort into new knowledge, approaches, and treatments.” The project will also lay the foundation for new prevention strategies and novel therapeutics, the director explained.
Finally, Collins pointed out that the NIH “has lost approximately 22 percent of its purchasing power for research since 2003.” Accordingly, the likelihood that a grant applicant will achieve funding after going through the peer review process “has fallen to the lowest in decades, now less than 20 percent.” The President’s budget request of $31.3 billion, $1 billion and 3.3 percent above the FY 2015 funding level, will put the NIH back on an increasingly stable trajectory. “We have never witnessed a time of greater promise for advances in medicine than right now. With your support, the future of medicine can be very bright,” Collins concluded.
Precision Medicine: One Million Person Cohort
Responding to Chairman Blunt’s inquiry of how the NIH intended to assemble the one million person cohort, Collins highlighted the recent creation of the PMI Working Group that met earlier in the week and will meet monthly between now and August. He announced that the Working Group’s next meeting will examine the question of what the ideal cohort is as it relates to demographics. The agency believes that it can use some of the cohorts that currently exist, including those put together by various health care delivery systems or the Veterans Administration (VA). There will undoubtedly be gaps in terms of representation and NIH wants to be certain that the cohort has the power to inform research on health disparities; the agency will need to figure out how to fill those gaps. The Working Group is expected to begin to make “strong recommendations” by August to allow the NIH to initiate the process of assembling this cohort.
Following up on Blunt’s question, Ranking Member Murray noted that the million person research cohort is very intriguing and emphasized that it needs to be “done right.” She asked Collins how the NIH will ensure that “it successfully represents all elements of the U.S. population, [including] women and minorities.” He replied that the question is exactly what the PMI Working Group is examining. It may require oversampling of certain minority groups to ensure that there is enough representation to have “powerful observations made possible about health disparities.” He added that the agency thinks of the participants of the study not just as subjects or patients, but participants and NIH partners. Collins estimated that it would take at least three or four years to put the entire cohort together, but expects that NIH will begin to learn from it before it is fully amassed.
Senator Lamar Alexander (R-TN), who chairs the Senate Health, Education, Labor, and Pensions (HELP) Committee, stated his intention to make the President’s proposal one of the Committee’s top priorities within its innovation efforts (see Update, March 24, 2015). He questioned how important “a properly functioning electronic medical record systems” will be to NIH’s effort to develop the cohort. Collins responded, “Enormously,” and explained that the agency is counting on using EHRs to make it happen.
Ranking Member of the full Appropriations Committee Barbara Mikulski (D-MD) shared that she and Blunt would like the subcommittee to visit NIH to allow for a more in-depth conversation about the agency. She then turned her attention to the President’s budget request for a $1 billion increase for NIH and asked Collins if that was enough, noting that the NIH had lost 20 percent of its purchasing power due to inflation since the doubling of its budget ended in 2003. Mikulski expressed her concern that despite Collins’ increase in management capability to set priorities, he is going to have to “end up picking winners and losers.” Acknowledging her support for the Precision Medicine Initiative, Mikulski emphasized her “worry about zip code medicine,” asking, “What is it that you truly need to do the job you need to do to serve America while we are trying to do ours?” Noting the reduction in purchasing power and cuts attributed to sequestration, Collins emphasized that the President’s $1 billion increase, would go a long way in putting the NIH back “on a stable upward trajectory.” The increase would allow the NIH to give 1,200 additional grants in FY 2016, added Collins.
Decreasing Rates of Dementia?
Murray also inquired about two studies, one in the U.S. and the other in Germany, which suggest the rates of dementia are is falling. Collins cautioned that there is reason to be skeptical whether one can be completely confident in the studies’ conclusions. To that end, the National Institute on Aging within the NIH is supporting two studies to conduct a rigorous epidemiological analysis to determine whether there is evidence of decreasing incidence or whether some of this is a diagnosis issue.
Early Stage Investigators
Murray also noted that since 2009, NIH has been monitoring the disparities between application success rates for experienced investigators versus early stage investigators and asked Collins what NIH is doing to level the playing field. NIGMS director Jon Lorsch explained that the agency is looking at various ways to address the problem. In addition to targeting the first application of new investigators, he shared the NIH’s concern that a critical stage is also individuals’ renewal application, a significant vulnerability for these individuals and one that the NIH will also examine. He further highlighted the new funding mechanism pilot which provides a single grant per investigator and the plan to introduce a version targeting new investigators (see Update, October 6, 2014).
Senator Tammy Baldwin (D-WI) expressed concern regarding the next generation of innovators and researchers and the significant gap in data on the existing research workforce. She also highlighted the lack of a comprehensive way to track the success of career researchers. She noted that her bill, the Next Generation Research Act, would ensure that NIH accelerates current and new policies to address the issue and foster new researchers. She requested more information regarding why there is not a good system already in place to track information about the biomedical workforce and what steps the NIH is taking to address the gap. Baldwin also inquired about NIH’s efforts surrounding chronic pain, opioid treatment, and alternatives.
Institutional Development Award
Senator Thad Cochran (R-MS) inquired about the NIH’s Institutional Development Award (IDeA) program. Lorsch responded that the program aims to ensure that cutting-edge biomedical research is being conducted in all 50 states. The NIH is committed to the program and thinks that it is an essential part of the NIH’s portfolio, he assured the Senator. Collins explained that the President’s budget request for the program maintains the FY 2015 funding level because the program received an exceptional increase between FY 2013 and FY 2014, and the agency is attempting to normalize its funding trajectory. This effort, however, should not be seen as a lack of enthusiasm for the program, he further assured Cochran.
Prioritization of NIH Research
Senator Jerry Moran (R-KS) pointed out that Congress has deferred to NIH when it comes to the prioritization of medical research, stating, “The theory has been that scientists should make the decisions about where the most promising opportunities are in finding the cure or the treatment.” He questioned whether the NIH “is making the best decisions possible to find cures that are the most readily available and most demanded by our citizens and the population of the world.” It is going to “become incumbent upon Congress to make decisions that are better made by you,” if the “NIH doesn’t do that prioritization,” Moran asserted. Collins acknowledged the concern and referenced the development of NIH’s overarching strategic plan covering all 27 institutes and centers to guide the agency’s priority decisions.
Reflecting on his time as a medical resident, Senator Bill Cassidy (R-LA) noted that the diagnosis of AIDS used to be a death sentence. He questioned the amount of funding dedicated to HIV/AIDS research, referencing a 2011 article that suggested that the principal variable in determining funding was disability-adjusted life years (DALYs). Collins explained that the NIH looks at the public health burden, and DALYs is a very well established way to do that. The agency also looks at scientific opportunity “because it is not going to be successful to throw money at the problem if nobody has an idea about what to do about it,” Collins stated. NIH also looks at what the peer review process is telling it about the “excellence of the science,” he explained further.
House Energy and Commerce Committee Releases Second Version of 21st Century Cures Draft
On April 29, the House Energy and Commerce Subcommittee on Oversight and Investigations released the second iteration of the Committee’s 21st Century Cures bill (see Update, February 24, 2015). The latest draft is a collaborative effort by Chairman Fred Upton (R-MI), Ranking Member Diana DeGette (D-CO), Energy and Commerce full committee Ranking Member Frank Pallone, Jr. (D-NJ), Health Subcommittee Chairman Joe Pitts (R-PA), and Health Subcommittee Ranking Member Gene Green (D-TX).
The new version of the discussion draft would authorize the National Institutes of Health (NIH) for three years (FY 2016 through FY 2018), and increase its funding level by $1.5 billion each year. It also creates an NIH Innovation Fund for FY 2016 through FY 2020, financed by a mandatory appropriation of $2 billion a year. The Innovation Fund would be used to support precision medicine, young emerging investigators, and other priorities yet to be identified.
The section-by-section summary of the discussion draft is available online here. A one-page summary is available online here.
ACS to Retain Marriage, Field of Degree Questions Proposed for Elimination
The Census Bureau will retain several questions in the American Community Survey (ACS) originally identified for removal: Person Question No. 12, undergraduate field of degree, and Person Question Nos. 21-23, which are related to marital history. The questions were proposed for elimination as part of the Bureau’s 2014 Content Review of the ACS and were released to the public for comment in the fall (see Update, November 3, 2014). COSSA objected to the removal of these questions in a written comment, as did many other organizations in the scientific community.
According to Census’ request to the Office of Management and Budget (OMB) for final clearance, it still plans to eliminate Housing Question No. 6, which asks if there is a business or medical office on the respondent’s property. The question was determined to have “no benefit to federal agencies, the federal statistical system, or the nation.”
The Bureau received 625 comments on Person Question No. 12, the field of degree question, coming from “researchers, professors and administrators at many universities, professional associations that represent science, technology, engineering and mathematics (STEM) careers and industries, members of Congress, the National Science Foundation, and many individuals interested in retaining this question.” The Bureau’s review of the comments concluded, “Given the importance of this small population group [STEM graduates] to the economy, the federal statistical system and the nation, bolstered by the new knowledge of historical precedent brought to light by commenters to the Federal Register notice, the Census Bureau therefore plans to retain this question on the 2016 ACS.”
More than 1,300 comments were received in favor of retaining the marital history questions. While the Bureau did not feel that the comments identified a federal regulation or law that requires collection of this information, the size of the response in itself was felt to be a significant argument in favor of keeping the questions.
As part of its ongoing efforts to better explain to the public why the ACS needs the information it collects, the Census Bureau has also released a new infographic, “Why We Ask.”
The Federal Register notice can be read in its entirety here. COSSA thanks all of the organizations who advocated for the importance of these questions to the social and behavioral science community.
White House Seeks Input on Using Science and Technology to Improve the Lives of Older Americans
In preparation for the 2015 White House Conference on Aging, the Office of Science and Technology Policy (OSTP) and the Domestic Policy Council are seeking input on science and technology initiatives that can improve the quality of life for older Americans. Examples of such activities might include:
- Expanded university and industry research and development to address challenges associated with aging
- Educational programs that help designers create “person-centered” products and services for older Americans
- Efforts to promote an “innovation ecosystem” for older Americans, which might involve accelerators, incubators, well-designed incentive prizes, and mechanisms for researchers and entrepreneurs to get rapid feedback on their proposed solutions
- The identification of one or more Grand Challenges around aging – ambitious but achievable goals that would significantly improve the health, independence, and quality of life for older Americans.
More information about the request for input is available here. Responses can be submitted through this form by May 22.
Eliseo J. Pérez-Stable Named Director of the National Institute on Minority Health and Health Disparities
O n April 28, National Institutes of Health (NIH) Director Francis S. Collins announced the selection of Eliseo J. Pérez-Stable, M.D. as Director of the National Institute on Minority Health and Health Disparities (NIMHD). He is expected to join NIH in September.
n April 28, National Institutes of Health (NIH) Director Francis S. Collins announced the selection of Eliseo J. Pérez-Stable, M.D. as Director of the National Institute on Minority Health and Health Disparities (NIMHD). He is expected to join NIH in September.
Currently at the University of California San Francisco (UCSF), Pérez-Stable is a professor of medicine, chief of the Division of General Internal Medicine, and director of the Center for Aging in Diverse Communities. He is also director of the UCSF Medical Effectiveness Research Center for Diverse Populations, which is addressing issues for African Americans, Asians, and Latinos in the areas of cancer, cardiovascular disease, and reproductive health. His personal research interest is in improving the health of poor and minority patients, reducing health risks such as smoking in minority populations, and improving cross-cultural communication skills among health care professionals.
Announcing Pérez-Stable’s appointment, Collins noted that “Eliseo is a highly respected leader with rich experience in advancing efforts to eliminate health disparities. He has the passion and vision to guide the NIMHD research agenda in this critically important area.”
Collins also recognized and thanked Acting NIMHD Director Yvonne T. Maddox for her “exemplary and dedicated service of leading the NIMHD efforts over the last year.” He also announced that Maddox is moving on to become Vice President for Research at the Uniformed Services University of the Health Sciences.
Back to this issue’s table of contents.
NIH: NIMHD Seeking Input for Health Disparities Science Vision
The National Institute on Minority Health and Health Disparities (NIMHD) within the National Institutes of Health (NIH) serves as the focal point for the agency’s conduct of research, research training, capacity-building, and outreach dissemination of minority health and health disparities. NIMHD recently initiated a scientific planning process in collaboration with the NIH institutes and centers designed to define a vision that will guide the development of “the science of health disparities research for the next decade and identify key research areas that should be given high priority because knowledge in those areas might inform translational efforts that could have a significant impact on reducing health disparities.”
To that end, NIMHD has issued a time-sensitive request for information (RFI), Soliciting Input into the NIH Science Vision for Health Disparities Research (NOT-MD-15-006). The Institute is seeking conceptual input regarding the science vision for health disparities research. Comments are being specifically sought regarding key research areas that might address the complexity of the multiple, interacting factors that often generate and perpetuate health disparities.
Research questions identified by NIMHD as important to the science vision process include, but are not limited to:
What are the causes of health disparities?
- What are the social, ecological, environmental and behavioral pathways, and the biological mechanisms that determinants of health operate upon to influence the health status of health disparity populations?
- How do different health determinants interact to produce health disparities? How can the complexity be captured while producing scientific information useful to guide policies and practice?
What are the best methods and metrics to study health disparities, their causes, and promising solutions? What measures, analytic approaches, and other methods will advance health disparities science?
- How should health disparities be measured in general, in physical health, in mental and psychosocial health, and in social health and wellbeing?
- Who should be the “reference” population in determining health disparities? Who should be compared to whom to measure health disparities? Should the criteria change over time in relation to the demographic and contextual changes, and if so, how?
- How can we leverage Big Data to determine the causes of health disparities and the pathways and mechanisms through which they operate?
- What methods should be used to evaluate the success of a health disparity intervention given the challenges often faced?
- How can we apply a population health systems approach to facilitate an understanding of the etiology of health disparities
What practice and policy interventions show the greatest promise to reduce and ultimately eliminate health disparities? What new knowledge is needed to inform effective interventions to address health disparities?
- What scientific research areas are most critical to study in order to inform pressing practice and policy questions addressing health disparities?
- What are the periods in the life cycle, timeframes or entry points along developmental trajectories that appear most promising as targets for interventions addressing health disparities across the life course?
What are the dissemination and implementation science approaches that will lead to effective practice and sustained policy intervention to reduce and eventually eliminate health disparities?
- What criteria should be used to determine whether a health disparities intervention is ready for dissemination and implementation? Can we develop systematic approaches for assessing “Implementation Readiness” of biomedical knowledge and interventions?
- How do we ensure that interventions are tailored to the needs of various health disparity populations while maintaining adequate fidelity of the intervention in a tested model?
Responses will be accepted through July 31, 2015 and must be submitted electronically to NIMHDScienceVision@mail.nih.gov.
NIH: Input Sought on Precision Medicine Cohort
The National Institutes of Health (NIH) is seeking feedback from the scientific community via a Request for Information (RFI): NIH Precision Medicine Cohort (NOT-OD-15-096) to guide it in creating a longitudinal cohort of one million or more Americans who have volunteered to participate in research as part of the President’s proposed Precision Medicine Initiative (PMI) (see Update, April 21, 2015).
Specifically, the agency is seeking information on characteristics, purpose, or other overall aspects in the development and implementation of a large U.S. precision medicine cohort. As participants in PMI, individuals will be asked to give consent for extensive characterization of biologic specimens and behavioral and environmental data, all linked to their electronic health records (EHRs). Qualified researchers will have access to the cohort’s de-identified data for research and analysis.
Precision medicine is defined as “the application of prevention and treatment strategies that take individual variability into account.” The NIH explains that this is not a new concept but opportunities for evidence-based precision medicine have greatly expanded as a result of the development of better large-scale biologic databases and computational databases, among other things. However, there is still a need for a research resource for developing and validating new approaches to precision medicine that “could be used to guide clinical practice ultimately to improve health.”
NIH’s goals for the NIH Precision Medicine Cohort are to enable better assessment of disease risk, understand disease mechanisms, and predict optimal therapy for a broad range of diseases through the study of a large group of people. These data will also enable observational studies of drugs and devices and potentially prompt more rigorous interventional studies that address specific questions. Participants may come from existing ongoing studies. In addition, participants may also be recruited de novo or be ascertained at random or by disease status.
The RFI emphasizes that characteristics of such a large-scale study that might maximize its research value may include:
- A sufficiently large number of participants to achieve adequate power for common disorders and reasonable representation of rare disorders;
- Intentional over-sampling of populations underrepresented in research to permit meaningful inferences about these groups and to study health disparities;
- A broad age range to provide information on disorders from infancy to old age;
- A broad range of genetic backgrounds and environmental exposures;
- A broad array of clinical and laboratory information, not limited to any single disease, as well as patient reported outcomes;
- Sophisticated dietary, other lifestyle, and environmental exposure assessment, preferably provided directly from participants using mobile devices and wearable sensors;
- Access to comprehensive electronic health data on participants for baseline, follow-up, and possibly also retrospective (prior to study entry) data collection, as well as return of actionable results for use in their clinical care;
- Return of appropriate information and results to participants as they desire;
- Collection and storage of biological specimens;
- Access to study data and biologic materials to qualified researchers to empower research on many diseases by researchers in many sectors;
- Community engagement in the design and implementation of the study, including a state-of-the-art consent process, to allow multiple uses of the data, regular feedback to participants about findings and progress; and
- A study design that ensures a high follow-up rate.
Therefore, the agency is seeking comments that address:
- Optimal study design and sample size for such a cohort.
- Data to be collected at baseline and follow-up, including mode of collection and frequency and length of follow-up.
- Potential research questions that could be uniquely or more efficiently and effectively pursued.
- Other suggestions for NIH to consider in the development and implementation of such a research cohort.
Finally, NIH is also interested in suggestions for existing or potentially new research entities that might be combined into a large U.S. cohort.
Responses to the RFI must be submitted online via its website: by May 7, 2015. For more information see the Notice. Additional information regarding PMI is available here.
APA Briefing Explains the Psychology behind False Confessions
The American Psychological Association (APA), a COSSA governing member, held a Congressional briefing on April 29, in conjunction with the Coalition for National Science Funding (CNSF) Exhibition. The briefing featured Saul Kassin, Distinguished Professor of Psychology at John Jay College of Criminal Justice (also a COSSA member), who spoke about his research on false confessions. Kassin observed that it is often difficult for people to understand why someone would admit to a crime they did not commit. However, in his analysis of a database of convictions overturned by DNA evidence, Kassin found that more than a quarter of the wrongly convicted individuals had made a confession.
Kassin’s talk was divided into two sections: “Why innocent people confess” and “Why confessions trump innocence.” In the first section, he described some of the harrowing experiences of individuals who had been wrongly convicted after confessing and explained some of the factors that put suspects at risk of making a false confession. These include vulnerability of the suspect, interrogation tactics like lying about evidence or prolonging the interview, and suspects’ belief that the fact their innocence will absolve them of charges, regardless of what they do or say during the police interrogation. In the second section of his talk, Kassin noted that for most people, a confession means that a person is guilty. However, few people can differentiate a false confession from a real one, and juries are overwhelmingly swayed by confessions—even in cases where they are told to disregard it. Furthermore, innocent people who have confessed are more likely to take a guilty plea than those who have not, because they know how strongly their confession will count against them in the courtroom.
Events Calendar
National Science Board Meeting, Arlington, VA, May 5-6, 2015
2015 Daniel Patrick Moynihan Lecture, Washington, DC, May 7, 2015
NSF Social, Behavioral, and Economic Sciences Advisory Committee Meeting, Arlington, VA, May 13-14, 2015
American Association for Public Opinion Research Annual Conference, Hollywood, FL, May 14-17, 2015
NSF Education and Human Resources Advisory Committee Meeting, Arlington, VA, May 19-20, 2015
Law and Society Association Annual Meeting, Seattle, WA, May 28-31, 2015
OBSSR 20th Anniversary Celebration, Bethesda, MD, June 23-25, 2015
OBSSR Capitol Hill Exhibition & Reception, Washington, DC, June 24, 2015
American Psychological Association Annual Convention, Toronto, Canada, August 6-9, 2015
Rural Sociological Society Annual Meeting, Madison, WI, August 6-9, 2015
American Statistical Association Joint Statistical Meetings, Seattle, WA, August 8-13, 2015
American Sociological Association Annual Meeting, Chicago, IL, August 22-25, 2015
A list of COSSA members’ annual meetings and other events can be found on the COSSA website. COSSA members who have an upcoming event they would like to see listed in the Events Calendar and on our website should send an email to jmilton@cossa.org.
Anti-Science COMPETES Bill Heads to House Floor
On April 23, the House Science, Space and Technology Committee passed along party lines (19 Republicans to 16 Democrats) the America COMPETES Reauthorization Act of 2015 (H.R. 1806). According to Committee Chairman and sponsor of the legislation Lamar Smith (R-TX), H.R. 1806 is a “pro-science and fiscally responsible bill.” It prioritizes basic research at the National Science Foundation (NSF), Department of Energy (DOE) Office of Science, and National Institute of Standards and Technology (NIST), while keeping funding levels within Congressionally-set discretionary spending limits. For NSF, the bill would increase funding for the Biological Sciences (BIO), Engineering (ENG), Mathematical and Physical Sciences (MPS), and Computer and Information Science and Engineering (CISE) directorates at the expense of other NSF accounts, including Social, Behavioral and Economic Sciences (SBE) and Geosciences (GEO). See COSSA’s analysis of H.R. 1806 for more information.
COSSA strongly opposes H.R. 1806 and issued a statement expressing our concerns.
Committee Ranking Member Eddie Bernice Johnson (D-TX) had harsh words for the bill during the more than five hour-long markup, noting that H.R. 1806 is the “combination of two bad bills” from last year, becoming a “doubly bad bill.” Further, she noted that the original America COMPETES Act enacted in 2007 and its reauthorization in 2010 were “landmark” pieces of legislation, vetted by dozens of scientific stakeholders through a transparent process. In contrast, H.R. 1806 was developed by Committee Republicans behind closed doors without federal agency or stakeholder input. In addition, while the previous two COMPETES bills aimed to ensure America’s preeminence in science and engineering, Johnson continued, the bill before the Committee “questions the motives of NSF and the integrity of scientists.” She expressed her embarrassment over the Committee’s consideration of the bill, noting that the nation would be better off with no bill than with H.R. 1806.
Johnson entered into the Committee record 30 letters (including COSSA’s) raising opposition or serious concerns with the legislation. In contrast, she noted that the previous COMPETES bills received hundreds of endorsements.
Research and Technology Subcommittee Ranking Member Daniel Lipinski (D-IL) called the targeting of SBE and GEO within H.R. 1806 a “partisan distraction” from what could otherwise be an important message on science, adding that the cuts to social science would be detrimental. He expressed his commitment to finding a bipartisan compromise, but added that he is unsure how to get there with this bill.
The Committee considered more than 30 amendments during the markup, most from the Committee’s Democratic members. About half of the amendments addressed concerns within the NSF title of the bill, including an amendment by Rep. Katherine Clark (D-MA) that would have struck the specific authorizations for NSF’s individual directorates, and amendments that would delete language tying NSF research to issues of “national interest” and misrepresentation of research results. These amendments were defeated along party lines.
Of particular note was an amendment in the nature of a substitute offered by Ranking Member Johnson, which took the form of a Democratic alternative bill to H.R. 1806 that was introduced on April 21, also called the America COMPETES Reauthorization Act of 2015 (H.R. 1898). Every Democratic member of Science Committee signed on to H.R. 1898 as original cosponsors.
Like the Republican bill that passed through the Committee today, the Johnson bill would authorize research efforts at NSF, DOE’s Office of Science, and NIST. However, that is where the similarities end.
The Johnson bill would authorize NSF for fiscal years (FY) 2016 through 2020; the Smith bill only provides authorizations for FY 2016-2017, requiring that the Committee turn back to NSF reauthorization in a year or so. In addition, the Democrats’ bill sets much more ambitious and sustained funding levels for the agency, with nearly 5 percent growth each year:
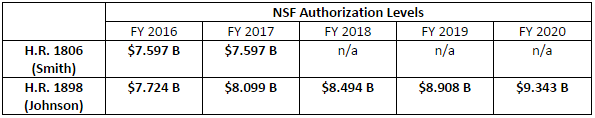
Further, the Johnson bill does not provide specific authorizations for NSF’s research directorates. Instead, it keeps with the current practice of providing an authorization for Research and Related Activities, Education and Human Resources, and other high-level accounts, and maintains NSF’s flexibility for determining how best to prioritize research funding.
The Johnson amendment in the nature of a substitute was defeated along party lines. Further the Science Committee is not expected to take up the Johnson COMPETES bill as a standalone measure.
H.R. 1806 now heads to the House floor for a vote. The bill’s predecessor, known as the FIRST Act in 2014, never received a floor vote. However, reports indicate that Chairman Smith is hoping to bring the bill to the floor in the near future, potentially as soon as next week.
Meanwhile, the Senate has not yet introduced COMPETES reauthorization legislation this year. However, Smith and Senate Commerce Committee Chairman John Thune (R-SD) issued a joint statement earlier today expressing their intent to work together on a COMPETES bill this year.
COSSA members and others can continue to weigh in on H.R. 1806 by writing to your Congressperson, especially as we head toward a potential floor vote in May.
Follow the action: #NOtoHR1806, #Stand4Science, @COSSADC

