Issue 14 (July 28)
COSSA Washington Update, Volume 34 Issue 14
Featured News
Congressional News
- Senate Committee Seeks Comments on COMPETES Revision
- FY 2016 Process Stalled as Congress Heads for Summer Break
- Senate Agriculture Appropriations Bill Advances through Committee
Federal Agency & Administration News
- NIH Seeks Input on Congressionally-Mandated Agency-Wide Strategic Plan
- NIH Seeks Comments on Proposed Alternative to National Children’s Study
Publications & Community Events
Funding Opportunity Announcements
COSSA Member Spotlight
Why Social Science? Share Your Stories!
 COSSA has launched a new campaign that seeks to collect stories of social science success from social and behavioral scientists across all disciplines. Is your research pushing the frontiers of science or advancing your field? Has your research contributed to an important finding or breakthrough? Are there interesting applications or potential applications to your work? If so, we want to hear it!
COSSA has launched a new campaign that seeks to collect stories of social science success from social and behavioral scientists across all disciplines. Is your research pushing the frontiers of science or advancing your field? Has your research contributed to an important finding or breakthrough? Are there interesting applications or potential applications to your work? If so, we want to hear it!
You may submit your stories using COSSA’s Why Social Science? webpage. Stories will be shared through social media (#WhySocialScience) and other COSSA outreach efforts over the next several months.
Senate Committee Seeks Comments on COMPETES Revision
On July 8, the Senate Commerce, Science and Transportation Committee issued a press release requesting public input to help guide the Committee’s development of America COMPETES Act reauthorization legislation. A follow-up release was issued last week providing additional details on the Committee’s plans. COMPETES is legislation originally enacted in 2007 that sought to ensure U.S. leadership in science and technology by making targeted investments at U.S. basic science agencies, including the National Science Foundation (NSF).
As COSSA has been reporting, the House introduced and ultimately passed its version of COMPETES legislation earlier this year with steep proposed cuts to social science research, despite ardent objection from the broad scientific community. The Senate has chosen a different approach for developing its NSF legislation this year, which according to the press release and discussions with Senate staff will include significant stakeholder involvement. Interested organizations are invited to submit written comment by August 21 addressing specific questions posed by the Committee.
FY 2016 Process Stalled as Congress Heads for Summer Break
Despite promises from Republican leaders in the House and Senate to pass fiscal year (FY) 2016 appropriations legislation through “regular order” this year, the FY 2016 process has stalled amid issues ranging from a policy rider pertaining to flying of the Confederate flag on federal grounds that killed the bills in the House and calls for the need to broker a larger budget deal. The House and Senate made some progress before the process sputtered out in recent weeks, with both chambers advancing all 12 of their bills through committee and the House managing to pass six of them; however, there were no FY 2016 bills brought to the Senate floor because of a united Democratic strategy to block the bills until a larger budget deal can be struck.
On the agenda upon Congress’s return in September will be to pass a continuing resolution (CR) to keep federal agencies operating beyond the start of the new fiscal year on October 1 and to avert a government shutdown. Rumors indicate that an initial CR could run until December. Also upon their return after Labor Day, Congress is expected to begin in earnest negotiations with the White House on a broader budget deal that will seek to provide some relief from sequestration, or the tight budget caps that are tamping down discretionary spending for defense and non-defense accounts, including research. Until these caps are addressed, and hopefully raised or eliminated, the FY 2016 appropriations bills remain in limbo with Senate Democrats expected to continue blocking the bills and President Obama noting his intent to veto any bill that keeps within the caps. All this makes for an expectedly busy fall for Congress.
AAAS Collecting Stories to Highlight the Importance of Scientific Conferences
The American Association for the Advancement of Science (AAAS) has launched a campaign to illustrate for policymakers the positive impacts scientific and technical conferences have on research. Over the past several years, federal regulations and policies have made it increasingly difficult for scientists and researchers employed by the federal government to attend conferences. AAAS is asking for stories that “highlighting the importance of conference participation to a healthy scientific and technical community,” particularly stories that involve collaboration with scientists from federal agencies, national labs, or research institutes. Stories may be submitted on the AAAS website.
Funding Opportunity Announcements
- NICHD: Innovative Development/Use of Technology to Increase HIV Testing and Linkage to Care Efforts in Adolescent Populations (R41/R42, R43/R44) (RFA-HD-16-029) (RFA-HD-16-030)
- NHLBI: Sickle Cell Disease Implementation Consortium (SCDIC): Using Implementation Science to Optimize Care of Adolescents and Adults with Sickle Cell Disease (U01, U24) (RFA-HL-16-010) (RFA-HL-16-011)
- NIAAA: Alcohol Use Disorders: Behavioral Treatment, Services and Recovery Research (R01, R03)
(PA-15-299) (PA-15-300)
COSSA Welcomes Virginia Tech
COSSA is thrilled to welcome Virginia Polytechnic Institute and State University (Virginia Tech) as its newest university member. Located in Blacksburg, VA, Virginia Tech receives more than $7 million annually in federal social and behavioral science research awards and is home to the Institute for Society, Culture and Environment.
COSSA’s full membership list can be viewed here. Interested in joining COSSA? More information here.
Events Calendar
- Live Long and Prosper: the Impact of Education on Mortality, Washington, DC, July 27, 2015
- Toxic Stress: How Economic Inequality Hurts Early Childhood Development, Washington, DC, July 30, 2015
- American Psychological Association Annual Convention, Toronto, Canada, August 6-9, 2015
- Rural Sociological Society Annual Meeting, Madison, WI, August 6-9, 2015
- American Statistical Association Joint Statistical Meetings, Seattle, WA, August 8-13, 2015
- American Sociological Association Annual Meeting, Chicago, IL, August 22-25, 2015
- National Conference on Health Statistics, Bethesda, MD, August 24-26, 2015
- American Political Science Association Annual Meeting & Exhibition, San Francisco, CA, September 3-5, 2015
- Economic History Association Annual Meeting, Nashville, TN, September 11-13, 2015
- Innovations in Research: Collaborations & Transformations, Cleveland, OH, September 16, 2015
- Council on Social Work Education Annual Program Meeting, Denver, CO, October 15-18, 2015
A list of COSSA members’ annual meetings and other events can be found on the COSSA website. COSSA members who have an upcoming event they would like to see listed in the Events Calendar and on our website should send an email to jmilton@cossa.org.
NIH Seeks Input on Congressionally-Mandated Agency-Wide Strategic Plan
The National Institutes of Health (NIH) recently issued a time-sensitive Request for Information (RFI) (NOT-OD-15-118) inviting comments and suggestions on the framework for its congressionally-mandated NIH-wide Strategic Plan. NIH is requesting feedback by August 16, 2015. The agency also plans to host webinars in early to mid-August to gather additional input.
NIH Deputy Director Larry Tabak presented the agency’s first iteration of its draft plan at the June 11 meeting of the NIH Advisory Committee to the Director (ACD) (see Update, July 14, 2015). At the July 20 meeting of the ACD, Tabak presented a revised draft framework incorporating the feedback it received from Committee members, including recommendations to keep the plan short, approximately 10 pages; make it inspirational and forward looking; integrate cross-cutting themes, and accentuate needed flexibility and nimbleness. The revised framework includes areas of opportunity that apply across biomedicine. Tabak explained that each area of opportunity will include a “succinct description of emergent opportunities” and will highlight specific examples of recent breakthroughs.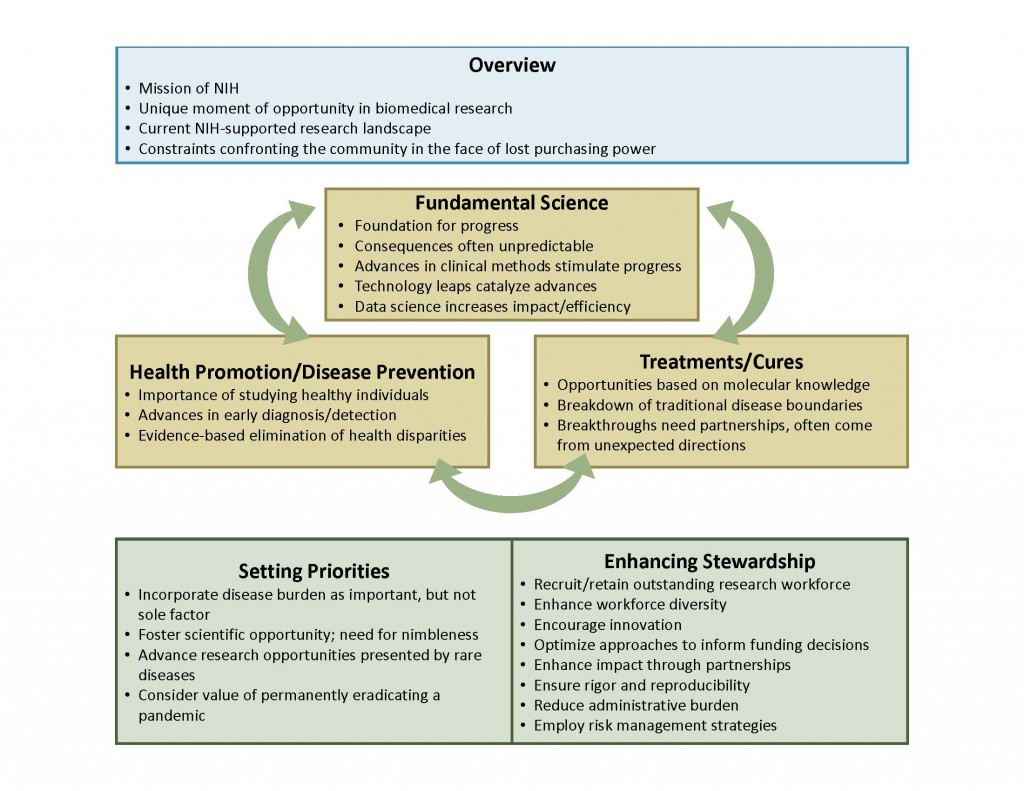 (more…)
(more…)
Senate Agriculture Appropriations Bill Advances through Committee
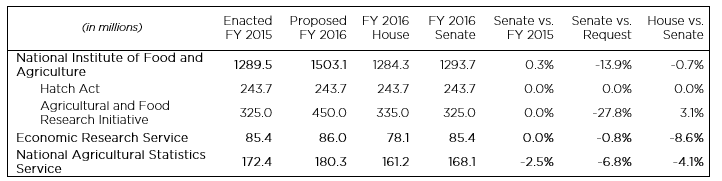
The Senate Appropriations Committee approved its version of the FY 2016 Agriculture, Rural Development, Food and Drug Administration, and Related Agencies Appropriations Bill (S. 1800) on July 16, after the bill’s approval by the Agriculture Subcommittee earlier in the week. Among the agencies funded in the bill are the U.S. Department of Agriculture’s (USDA) principal statistical agencies, the Economic Research Service (ERS) and National Agricultural Statistics Service (NASS), and the National Institute of Food and Agriculture (NIFA), which houses the Department’s main competitive grants program, the Agriculture and Food Research Initiative (AFRI). The House Appropriations Committee passed its version of the bill on July 8 (more on the House bill is available here).
In general, the Senate bill would provide more funding to agencies of interest to the social and behavioral science community than the House bill. However, the Committee’s adherence to current spending caps means that the proposed funding levels still fall below the Administration’s request. The bill would maintain ERS’ FY 2015 funding level of $85.4 million, $7.3 million above the cut proposed by the House. NASS would be cut by $4.3 million compared to FY 2015, but the Senate mark is still $6.9 million more than the House bill. In the committee report, both agencies are encouraged to continue to collect and analyze data on organic agriculture.
Under the Senate bill, NIFA would receive a small increase over FY 2015, but the total falls well short of the Administration’s proposed funding level of $1.5 billion. AFRI would actually receive $10 million less under the Senate’s bill compared to the House version—the same amount as in FY 2015.
Neither bill is likely to reach the floor of either chamber anytime soon as the larger debate over sequestration and spending caps has stalled the FY 2016 process and likely will not be resolved until later in the fall at the earliest.
NIH Seeks Comments on Proposed Alternative to National Children’s Study
The National Institutes of Health (NIH) is inviting comments on its proposed plan for the Environmental influences on Child Health Outcomes (ECHO) program and has issued a time-sensitive Request for Information (RFI).
Background
The ECHO program responds to the NIH’s decision to discontinue the National Children’s Study (NCS) in December 2014, per the recommendations of a working group of the Advisory Committee to the Director (ACD) of NIH (See Update, December 19, 2014). NIH emphasizes that in keeping with the spirit of the NCS, ECHO aims to address the critical goal of understanding the impact of environmental influences on children’s health and development.
The NCS was originally authorized by the Children’s Health Act of 2000. A program office within the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) led the implementation efforts of the study. The NCS was intended to be a “longitudinal observational birth cohort study to evaluate the effects of chronic and intermittent exposures on child health and human development in U.S. children.”
In December 2014, NIH director Francis Collins announced that per the recommendations of an Institute of Medicine report and the subsequent ACD recommendations the agency would discontinue the study. Congress, however, did not receive the decision as well as NIH would have hoped. At the March 2015 Labor-HHS hearings, Rep. Lucille Roybal-Allard articulated Congress’ concern with the decision: “Congress fully expected that the study would be carried through to its completion… In almost every fiscal appropriations report from the year 2000 to 2014 there have been specific instructions from both the House and the Senate directing the continuation of the Study.”
Accordingly, in its report accompanying the fiscal year (FY) 2016 appropriations bill for the Departments of Labor, Health and Human Services, Education, and Related Agencies (Labor-HHS), the House expressed “disappointment” with the agency’s decision to discontinue the study. Similarly, the Senate Appropriations Committee found NIH’s decision to discontinue the National Children’s Study (NCS) after $1.3 billion in federal investment “deeply troubling” (See Update, June 30, 2015). Nevertheless, both houses continue to provide the resources to continue to fund research that would have been supported by NCS.
The House Appropriations Committee included report language that “directs and provides funding for continuation of the NCS in an alternative form called the National Children’s Study Alternative (NCS-A).” The House further directs NIH to work with pediatric groups to “develop a series of alternative research activities that build on NCS data and the overarching goals of the NCS to address the developmental origins of health and disease through a series of studies (including longitudinal) that incorporate expertise in biology and epidemiology, integrate basic science, and leverage maternal/infant cohorts, either de novo or from extant networks.” The agency is expected to focus on “at least prematurity, obesity, autism, asthma, and pediatric, rare diseases like cancer.” NIH is urged by the Senate “to recalibrate and realign the investment already made in the NCS to initiate new and focus existing longitudinal studies to address the objectives identified for the NCS. The NIH should rely upon a formal scientific advisory mechanism to coordinate efforts across studies. The research efforts should incorporate expertise in population health and environmental epidemiology, integrate basic science, and leverage maternal/infant cohorts.”
Request for information
In the RFI notice, NIH explains that to make the best use of the FY 2015 appropriated funds, the agency has identified opportunities to address “challenges at the intersection of pediatric and environmental health through alternative approaches that are consistent with the original goals of the NCS, including establishing compelling new programs, integrating existing programs, and enhancing programs by incorporating more comprehensive environmental assessments.” A major focus of this new effort is on “the development of tools to enhance measurement of environmental exposures (e.g., physical, chemical, biological, psychosocial) and facilitate research across all of the initiatives and programs.” The notice also notes that a second “key component of the plan is studying environmental influence on placental and in utero development, with the goal of identifying the “seeds” of future diseases and conditions.”
The agency also explains that the overarching goal of ECHO is to investigate the longitudinal impact of prenatal, perinatal, and postnatal environmental exposures on pediatric health outcomes with high public health impact by leveraging and expanding extant cohorts. To this end, NIH proposes to support “multiple synergistic, longitudinal studies using extant cohorts that represent variable environmental exposures (e.g., physical, chemical, biological, psychosocial, natural and built environments) that will share standardized research questions and focus on four key pediatric outcomes – upper and lower airway; obesity; pre-, peri-, and postnatal outcomes; and neurodevelopment.”
According to the RFI, all longitudinal studies will collect the same standardized, targeted data (Core Elements) as a component of the project, and will be managed through a Coordinating Center. An additional opportunity for creating an Institutional Development Award (IDeA) States National Pediatric Clinical Research Network is also being considered which would be expected to address access gaps for rural children through a national network for pediatric research embedded at IDeA locations and link existing IDeA state centers with experts in clinical trials.
In addition to comments on research questions specific critical pediatric conditions, NIH is also seeking comments on any or all of, but not limited to, the following topics: potential benefits, drawbacks, and areas of consideration for leveraging existing cohorts to collect standardized data elements; additional core elements to be considered; considerations for harmonizing data across cohorts; high impact areas of opportunity in addition to those listed; and anticipated advances and/or considerations for implementing state-of-the-art data collection and analytic methodologies throughout the duration of the study.
The deadline for responding to the agency’s request is August 15, 2015. All comments must be submitted electronically on the NIH’s website.